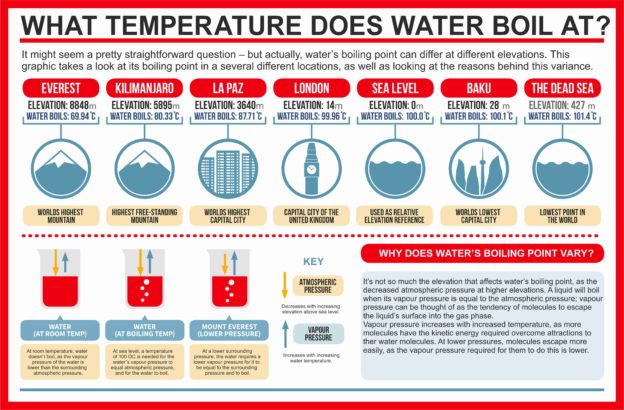
Promethium
The history of one rare–earth element is so unusual that it merits individual discussion. Promethium, as it is known now, is practically non–existent in nature (we write practically but not absolutely and the reason for that will be clear later). Event which can only be described as amazing preceded the discovery of element 61 by means of nuclear synthesis.
The work of Moseley made clear the existence of an unknown element between neodymium and samarium. But the situation proved to be not so clear and dramatic events rapidly followed in the history of element 61.
The New World was unlucky in discoveries of new elements. All the elements known by the twenties of this century (not counting the elements known from ancient times) had in fact been discovered by the European scientists. This is why the American scientific community was particularly happy to learn about the discovery of element 61 by the chemists from Chicago B. Hopkins, L. Intema, and J. Harris in 1926.
Starting from 1913 scientist from various countries had been searching intensely for the elusice rare–earth element and it seemed strange that they had not found it earlier. Indeed the elements of the first half of the rare–earth family known as the cerium elements (from lanthanum to gadolinium) had been shown by geochemists to be more abundant in nature than the yttrium elements of the second half of the family (from terbium to lutecium). But all the yttrium elements had been found while an empty box had remained in the cerium group between neodymium and samarium.
The straightforward explanation was that element 61 was not just rare but rarest element. Its abundance was assumed to be much lower than that of other rare–earth elements, and the available analytical techniques were not sensitive enough to identify its traces in the terrestrial minerals. New more sensitive methods were needed for the purpose.
The American chemists employed X–ray and optical spectral techniques to study the minerals where they hoped to find element 61. These well versed in the history of range earth elements could say that the path the Americans took was a troublesome one as spectral analysis not infrequently had acted as an evil genius of rare–earth studies despite all the benefits it had brought to them. But in the twenties the feet spectroscopy stood on were not so unsteady as a few decades earlier and the Moseley law could be used for predicting the X–ray spectra of any element.
The American chemists worked hard, analysed numerous specimens of various minerals and in april 1926 reported the discovery of element 61. But they did not extract even a grain of the new element and its existence was inferred from the X–ray and optical spectral data.
The discoverers University named the element illinium in honour of the Illinois University where they worked and the symbol Il took its place in box 61 of the periodic system but just a half–year later a new claimant of box 61 came into the limelight. It had been discovered by two Italian scientists L. Rolla and L. Fernandes who had named it florencium (Fl). Allegedly, they had discovered element 61 two years earlier than the Americans but failed to report the discovery owing to some undisclosed reasons. They had sealed the report of their discovery into an envelope and left it for safe–keeping in the Florence Academy.
If different people obtain the same result with different means that would seem to prove that the result is genuine. Americans and Italians could be only too happy. As for the question of priority it was nothing new to science. But no one of the alleged discoverers of element 61 could imagine that their argument about periodic would soon become superfluous and both symbols, Il and Fl, would be shown to be illegal squatters in box 61 of the periodic table.
To trace the events now we have to go not further but some time back to the facts that were simply unknown at the time. The report of the discoverers of element 61 started with the words: “There had been absolutely no grounds for assuming the existence of an element between neodymium and samarium until it was demonstrated through the Mosely law”. Typical dry style of a scientific report, everything would seem to be correct. But….
The following remarkable conclusion in German (please, do not look it up in a dictionary yet) appeared in the margin of a hard–written manuscript of the element table found in the papers of certain scientist (we shall supply the name a little later): “NB. 61 ist das von mir 1902 vorhergesagte fehlende Elemente”.
The real history of element 61 should prominently feature the name we have already met on these pages. It is the Czech scientist Boguslav Brauner, Mendeleev’s friend and an eminent expert in the chemistry of rare–earth elements.
Illinium had been discovered, the discoverers accept congratulations and learn about the second, third, fourth confirmation of the discovery from the scientists of other countries. The pedigree of element 61 could be started thus: “Moseley had predicted and American chemists discovered”. But a discordant not unexpectedly sounded in November 1926 from the pages of Nature. It was none other than Brauner. He congratulated him American colleagues but voiced his disagreement with the above–cited beginning of their report. He argued that it was really not important who first discovered element 61 –American or Italians; in the twenties scientists became increasingly aware that the discovery by itself was a purely technical matter. The important issue is who predicted the new element. Was it Moseley? No, declared the Czech scientist. Who then? Of course, he himself, Boguslav Brauner…..
But nothing could be further from the truth if we thought that he was immodest. His claim was based on his vast experience of work with rare earths, on his profound understanding of the spirit of the periodic system, on his superb appreciation of slight changes of properties in the series of extremely similar rare–earth elements, and, finally, on his intuition of a dedicated researcher.
But these words of praise must be substantiated with facts. Let us turn back to 1882. The old didymium of K. Mosander is close to its death. P. Lecoq de Boisbaudran had already extracted a new element, samarium, from it. B. Brauner carefully analyses the residue and employing extremely complicated chemical procedures separates it into three fractions with different atomic masses. Owing to a number of reasons he has to discontinue his work and in 1885 K. Auer von Welsbach overtakes the Czech scientist. The old didymium is dead but praseodymium and neodymium have appeared, the first and the third fractions of Brauner. But what about the intermediate second fraction? No, its tine has not come. The chemistry of rare–earth elements is in a turmoil. The muddy stream of erroneous discoveries of new elements overflows with doubts the very periodic system. But life goes on. The chaos in rare earths gradually diminishes and the known rare–earth elements form an ordered series. Now Brauner notices that the difference between the atomic masses of neodymium and samarium is rather large; it is larger than the respective difference between any two neighbouring rare–earth elements. His brilliant knowledge of rare earths suggests to Brauner that there is a discontinuity in the variations of their properties in the part of the series between neodymium and samarium. At last, he recalls his work of 1882. The clues fit into a pattern leading to premonition and even certainty that an unknown element can be found between neodymium and samarium. But as his friend, Mendeleev, Brauner was never too hasty in his conclusions. It was only in 1901 that he placed an empty box between neodymium and samarium when he put forward his views on the place of the rare–earth elements in the periodic system.
Now we can give a translation of the note he wrote in margin of his hand–written table of elements. It reads: “61st element is the missing element predicted by me in 1902”.
His short letter to Nature was an attempt by Brauner to put the record straight. This would seem to simplify the task of science historians in writing the history of element 61. But a history is meaningful only if it treats a subject which really exists. As for illinium the element proved to be still–born.
While the hotheads kept trying to squeeze the symbol Il into box 61 of the periodic table meticulous critics tried to verify the discovery. The careful experiments by the first of them, Prandtl, could be doubted by nobody. But his results did not even hint at the existence of element 61.
In 1926 the Noddacks who had just announced their discovery of masurium and rhenium (Nos. 43 and 75) started their tests. They used all available techniques to analyse fifteen various minerals suspected of containing illinium. The processed 100 kilograms of rare–earth materials and could not detect a new element. The Noddacks claimed that if the American’s results had been correct they, the Noddacks, would undoubtedly extracted the new element. Even if the element were 10 million times rarer than niodymium or samarium they would still find it… There are two possible explanations: either element 61 is so rare that the existing experimental techniques are not fine enough to find it or wrong mineral specimens were taken.
Geochemists were against the first explanation. The abundances of rare–earth elements are more or less similar. There are no reasons to think that illinium is an exception. They suggested looking for illinium in minerals of calcium and strontium. All rare–earth elements are typically trivalent but some of them can exhibit a valence of two or four. For instance, europium rather easily gives rise to cations with a charge of two. Their size is closer to those of calcium and strontium cations and they can replace the letter in the respective alkaline–earth minerals. Perhaps, illinium has a similar more pronounced capacity and can be found in some rare natural compound of strontium. One hypothesis replaced another, one assumption stemmed from another, unsubstantiated one. Just in case, the Noddacks analysed several alkaline–earth minerals. Alas, they failed once more.
The search for illinium seemed to come to a dead end; though it still went on the reported results were little believed. Chemists failed in looking for element 61 in the terrestrial minerals it was theoretical physics whose fate it was to open up the “envelope” where nature had “sealed” element 61. But when the envelope was open the scientist (not for the first time!) were disappointed. The envelope was empty.
At this point the fate of element 61 directly involves the fate the element 43, that is, technetium. According to the law formulated by the German theoretical physicist Mattauch, technetium in principal cannot have stable isotopes. This law also forbids existence of stable isotopes of element 61. Illinium is dead but element 61 must survive.
But what if it really does not exist? I. Noddack put forward a daring idea that illinium (we shall use this name for the time being) had existed on Earth in early geological periods. But it had been a highly radioactive element with a short half–life and it had decayed fairly soon and disappeared from the face of Earth. If we agree with this idea we have to make two extremely unlikely assumptions. First, illinium which is at the centre of the periodic table has no stable isotopes. Second, the half–lives of its isotopes a e much shorter than the age of Earth.
Indeed, illinium neighbours in the periodic system (neodymium and samarium) have many (seven each) natural isotopes with a wide range of mass numbers–from 142 to 154. Any feasible isotopes of element 61 would have its mass number in this range. Thus, any illinium isotopes proves to be unstable in this range of mass numbers. The Mattauch law seem to bury for good the hopes to find element 61 on Earth. But then a gleam of hope appeared. All right, the illinium isotopes are all radioactive. But to what extent? Perhaps the half–lives of some of them are very long. At that time the theory had not learned how to predict half–lives of isotopes. The search for element 61 had to continue in the dark. Physicists believed that only nuclear synthesis could solve the riddle of element 61 the more so as the case of technetium was fresh in their minds.
As if trying to restore the honour of American science after its setback in 1926 two physicists from the University of Ohio conducted the first experiment of artificial synthesis of element 61 in 1938. They bombarded a neodymium target with fast deuterons (the nuclei of heavy hydrogen). They believed that the resulting nuclear reaction Nd + d → → 61 + n gave rise to an isotopes of element 61. Their results were inconclusive but nevertheless they thought that they obtained an isotope of the new element with the mass number of 144 and the half–life of 12.5 hours.
Again sceptics said that these results were erroneous and not without a reason since nobody could be sure that the neodymium target was ideally pure. The method of identification could hardly be considered reliable, too. Even uncomplicated optical and X–ray spectra evidenced the presence of element 61 as in the study of 1926; the conclusion was made from the radiometric data.
In fact, chemistry was not involved in this work and the chemical nature of the mysterious radioactive product was not determined. Therefore, one may ask whether 1938 can be regarded as the actual data of discovery of element 61. It can rather be said that only the consistent efforts to synthesize it started at the time.
As time passed the range of bombarding particles was extending, targets of other rare–earth elements were used, and the techniques of activity measurements were improved. Reports on other illinium isotopes started to appear in scientific journals. Element 61 was becoming a reality albeit an artificially created one. Its name was changed to cyclonium in commemoration of the fact that it was produced in a cyclotron but the symbol Cy did not remain for long in box 61 of the periodic table.
Researchers had detected the radioactive “signal” of cyclonium but nobody had seen even a grain of the new element and its spectra had not been recorded. Only indirect evidence of the existence of cyclonium had been obtained.
The history of science of the 20th century knows of many great discoveries and one of the greatest is the discovery of uranium fission under the effect of slow neutrons. The nuclei of uranium–235 isotopes are split into two fragments, each of which is an isotope of one of the elements at the centre of the periodic table. Isotopes of thirty odd elements from zinc to gadolinium can be produced in this way. The yield of the isotopes of element 61 has been calculated to be fairly high–approximately 3 per cent of the total amount of the fission products.
But the task of extracting the 3 per cent amount proved to be very difficult. The American chemists J. Marinsky, L. Glendenin, and Ch. Coryell applied a new chemical technique of ion–exchange chromatography for separation of the uranium fission fragments.
Special high–molecular compounds known as the ion–exchange resins are employed in this technique for separating elements. The resins act as a sieve sorting up elements in an order of the increasing strength of the bonds between the respective elements and the resin. At the bottom of the sieve the scientists found a real treasure–two isotopes of element 61 with the mass numbers 147 and 149.
At last, element 61 known as illinium, florencium, and cyclonium could be given its final name. According to recollections of the discoverers, the search for a new name was no less difficult than the search for the element itself. The wife of one of them, M. Coryell, resolved the difficulty when she suggested the name promethium for the element. In an ancient Greek myth Prometheus stole fire from heaven, gave it to man and was consequently put to extreme torture by Zeus. The name is not only a symbol of the dramatic way of obtaining the new element in noticeable amounts owing to the harnessing of nuclear fission by man but also a warning against the impeding danger that mankind will be tortured by the hawk of war, wrote the scientists.
Promethium was obtained in 1945 but the first report was published in 1947. On June 28, 1948, the participants at a symposium of the American Chemical Society in Syracuse had a lucky chance to see the first specimens of promethium compounds (yellow chloride and pink nitrate) each weighing 3 mg. These specimens were no less significant than the first pure radium salt prepared by Marie Curie. Promethium was born by the great creative power of science. The amounts of promethium prepared now weigh tens of grams and most of its properties have been studied.
The Mattauch law denied the existence of terrestrial promethium but this denial was not absolute. The search for promethium in terrestrial ores and minerals would be quite in order if promethium had long–lived isotopes with half–lives of the order of the age of Earth.
But in this respect nuclear physics proved to be a foe of natural promethium. With each newly synthesized promethium isotope a possible scope for search became increasingly narrow. The promethium isotopes were found to be short–lived. Among the fifteen promethium isotopes known today the longest–lived one had a half–life of only 30 years. In other words, when Earth had just formed as a planet not a trace of promethium could exist on it. But what we mean here is the primary promethium formed in the primordial process of origination of elements. What was discussed was the search for the secondary promethium which is still being formed on Earth in various natural nuclear reactions.
Technetium was finally found on Earth among the fragments of spontaneous fission of uranium. These fission products could contain promethium isotopes. According to estimates, the amount of promethium that can be produced owing to spontaneous fission of uranium in the Earth’s crust is about 780 g, that is, practically, nothing. To look for natural promethium would be tantamount to dissolving a barrel of salt in the lake Baikal and then trying to find individual salt molecules.
But this titanic task was fulfilled in 1968. A group of American scientists including the discoverer of natural technetium P. Kuroda managed to find the natural promethium isotope with a mass number of 147 in a specimen of uranium ore (pitchblende). This was the final step in the fascinating history of the discovery of element 61.
As in the case of technetium, we can name two dates of discovery of promethium. The first date is the date of its synthesis, that is, 1945. But under the circumstances synthesis was unconventional (it could be called fission synthesis). The first two promethium isotopes were extracted from the fragments of fission of uranium irradiated with slow neutrons rather than in a direct way as was the case with technetium, which was produced in a direct nuclear reaction. This makes promethium a unique case among all over synthesized elements.
The second date is the date of the discovery of natural promethium, that is, 1968. This achievement is of independent significance as it stretched to the utmost the capabilities of the physical and chemical methods of analysis. Of course, the achievement is of a purely theoretical significance since nobody can hope to extract natural promethium for practical uses.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()



![Rendered by QuickLaTeX.com \[\begin{align} & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,N{{H}_{2}} \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,/ \\ & 2Hg_{2}^{2+}+NO_{3}^{-}+4N{{H}_{3}}+{{H}_{2}}O\to HgO.Hg\,\,\,\,\,\,\,\downarrow +2Hg\downarrow +3NH_{4}^{+} \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\backslash \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,NO \\ \end{align}\]](https://thechemistryguru.com/wp-content/ql-cache/quicklatex.com-d64edb84c37d600cb06bb3b36d3cb695_l3.png)