Bonding in Carbon
In general carbon involves in covalent bonding.
Covalent bond:
A bond formed by the sharing of valence electrons between atoms of similar electronegativity is called covalent bond.
Properties of covalent compounds:
Covalent compounds have low melting and boiling points.
Covalent compounds are non conductors of electricity this is due to the absence of free ions.
Classification of covalent bonds:
Based on the number electrons shared between the atoms, the covalent bond is classified into three types. They are:
- Single covalent bond
- Double covalent bond
- Triple covalent bond
Single Covalent Bond:
Single covalent bond is formed by sharing a single pair of electrons.
Example: H2 (H – H)
Hydrogen has one electron and it requires one more electron to attain the nearest inert gas configuration. To achieve this each hydrogen atom contributes an electron to form a single bond. Thus, a single covalent bond is formed between the two atoms of the hydrogen molecule.
Double Covalent Bond:
Double bond is formed by the sharing of two pairs of electrons of the valence shell.
Example: Oxygen molecule (O = O)
The atomic number of oxygen is 8. It requires two electrons to achieve the nearest stable inert gas configuration, which is neon. To achieve this, two oxygen atoms contribute two unpaired electrons to produce two bond pairs. Thus, they share these two electron pairs to form a double bond

Triple Covalent Bond:
Triple bond is formed by sharing of three pairs of electrons of the valence shell.
Example : Nitrogen molecule (N ≡ N).
The atomic number of nitrogen is 7. It requires 3 more electrons for attaining the nearest inert gas (neon) configuration. Thus, 2 nitrogen atoms combine together and produce 3 bond pairs and share the three bond pairs between them.

Representation of a Bond:
Bond formation can be represented using Lewis structures. The Lewis dot structures provide a picture of the bonding in molecules in terms of the shared pairs of electrons and the octet rule.
Example: Lewis dot structures of CCl4 and CH4.
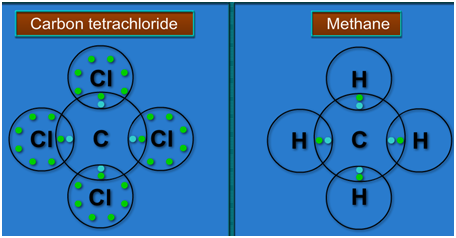
Bond Formation in Carbon:
From the electronic configuration of carbon, it is clear that it has to either gain or lose four electrons, to attain noble gas configuration.
If carbon gains four electrons it would form a C–4 ion. It is unable to hold four extra electrons. It would be difficult for the six protons to hold the ten electrons.
Formation of C+4 is difficult as it requires high amount of energy which leaves six protons in the nucleus and holding only two electrons.
Thus formation of both C–4 and C+4 forms is difficult.
Carbon overcomes this difficulty by sharing its electrons with other atoms of carbon or with atoms of other elements. Sharing of electrons results in a covalent bond and the shared electrons belong to either of the atoms, this sharing helps in achieving noble gas configuration.
Covalent Bond Formation in Methane:
During the formation of a methane molecule carbon atom share its four valence electrons with four hydrogen atoms. Thus in methane molecule there exists four single bonds between carbon and four hydrogen atoms.

Double bond formation of Carbon:
Carbon can involve in double bonding either with it self or with other atoms like Oxygen.
Example:
Formation of CO2: Carbon shares its four valence electrons with two oxygen atoms. Thus carbon forms two double bonds with two oxygen atoms.
![]()
Triple bond formation of Carbon:
Carbon can involve in triple bonding either with it self or with other atoms like Nitrogen.
Example:
Formation of HCN: Carbon shares three of its valence electrons with one nitrogen atom to form triple bond with it. And shares one electron with one hydrogen to form single bond with it.
![]()
